Supply Chain projects are often initiated for compliance reasons, but they become truly transformational when you consider how existing technologies and workflows can be optimized to deliver ongoing business value.
By viewing your supply chain challenges in this way and by using our flexible Strategy through Sustainability methodology, we deliver cost-effective and scalable solutions that are right-sized for the individual use cases and requirements of your business.
Our Supply Chain Consulting services include:
Serialization Compliance
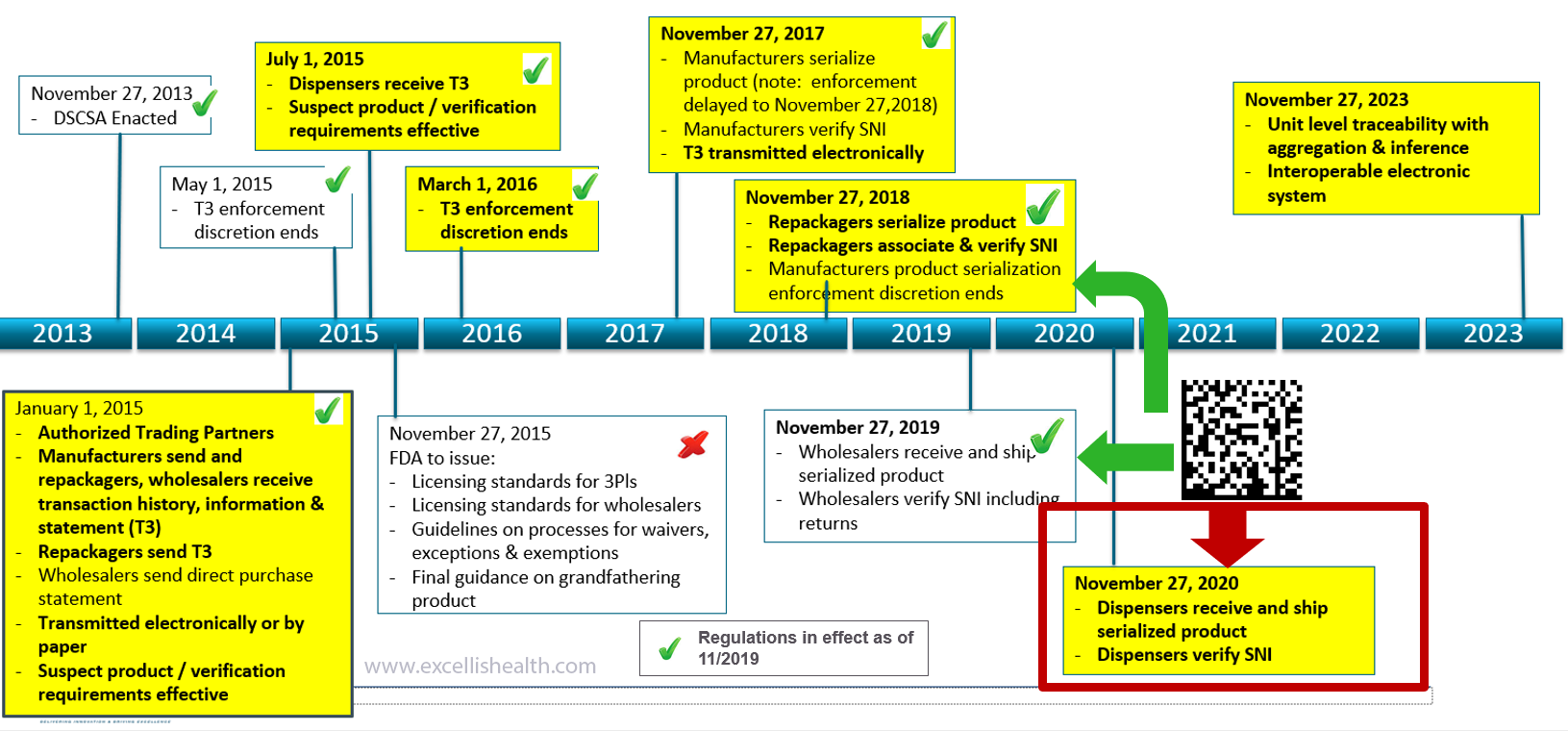
Our tailored serialization programs ensure your supply chain is fully compliant – and stays that way. From the Drug Supply Chain Security Act (DSCSA) and Europe’s Falsified Medicines Directive (EU-FMD) to similar regulations in emerging markets like Russia, Saudi Arabia, Brazil, and China, we are the undisputed global leader in serialization for every segment of the pharmaceutical supply chain.
Ready for Compliance?
Working from Strategy through Sustainability across every possible use case, we provide a variety of Packaged Services and Implementation Resources to outsource or complement your serialization compliance efforts. We also help operationalize your serialization investment, ensuring that if you want to create value beyond compliance, you have a rock-solid foundation to build on.
-
Launching your first product in the US?
-
Taking a US-approved product to Europe?
-
Evaluating if and how to enter other global markets?
-
Deciding if you need a Verification Router Service (VRS) for US Saleable Returns?
-
Looking ahead to full aggregation and/or serialized warehousing and distribution?
If you answered YES to any of those questions, contact us to help ensure serialization compliance does not slow you down.
Serialization Value
Not only will we ensure your business complies with new and existing regulations, we offer a range of services that extend beyond compliance.
We are already working with a number of best-in-class companies to deliver strategies and solutions that drive ongoing value across multiple segments of the supply chain.
These include:
-
Product visibility and inventory management
-
Recalls, chargebacks, shortages, indications, and efficacy
-
Patient health and/or consumer engagement
-
Integration with ordering and receiving systems
-
Data aggregation, management, analytics, and monetization
-
Accreditation, contracting and reimbursement for retail, mail-order, and specialty services

EU/ROW Go-To Market
Excellis Europe is the world’s leading pharma supply chain and serialization experts. We combine rich experience with world-class expertise to deliver actionable intelligence.
As an industry partner of leading fortune 200 companies we strategically support serialization journeys for MAHs, CMOs, distributors and re-packagers. We fully understand the vertical markets of the pharma industry and this experience in the domains of regulatory, manufacturing, ERP, EPCIS, MES, warehouse, supply chain and validation that will get you compliant in time and on budget.
Some of the services we offer:
-
Russia Traceability Solutions
-
CMO On-boarding
-
Passport Service
-
Alert Management
Enterprise Resource Planning
We are a certified partner to the world’s leading ERP platforms and have extensive experience with SAP, Oracle, JD Edwards (JDE), QAD, Microsoft Dynamics, Great Plains, and NetSuite.
From strategy and implementation through sustainability, we can help you “right size” an existing or future ERP that meets your supply chain needs in both the short- and long-term.
Our ERP services include:
-
Strategy through Sustainability Methodology
-
Project Management
-
Validation
First Time Launchers
Excellis guides organizations through the First-Time launch process by leveraging people, processes, and right-sized technologies. Our methodology ensures the success of your first commercial launch by leveraging a proven playbook that eliminates risk and maximizes readiness for all pre and post launch activities.
Many small biotechnology and pharmaceutical organizations historically followed a standard launch path for their assets by exploring out-licensing, partnerships, and outright acquisition. In recent years, more and more of these companies are launching their new drug themselves, rather than relying on large pharma companies to do it for them. The key question to this process is – how can they get it right the first time?
Brand Protection
Excellis helps brand owners protect their patients/consumers, brands, and business from risk related to illicit trade, to include counterfeiting, grey market/diversion, and product tampering. It’s a risk businesses can no longer afford to ignore.
Excellis has been building programs to protect supply chains since 2010. With early roots in regulated industries, Excellis has worked globally to enable people, process, and technology know-how to provide clients with compliance and assurance solutions. Whether you’re at the earliest stage of program exploration or you’re leading a mature program and looking to address gaps and opportunities, we can help.
Quality & Validation
Our cost-effective range of validation, qualification, and testing solutions help you improve your systems and processes to reduce variability, mitigate the risk of non-compliance and ensure reliability. We also offer a range of quality management services to ensure your systems are optimized and compliant from the very beginning of your journey towards commercialization. This helps mitigate your risk of experiencing costly and/or time-consuming non-compliant situations when going to market, while at the same time driving process reliability and repeatability.
Whatever sector you operate in, our experienced subject matter experts use proven processes and industry best practices to help you comply with all necessary regulations and drive value across your supply chain.
Some of our services include:
-
Computer Systems Validation (CSV)
-
Process and Method Validation
-
Quality Health Check
-
Quality Systems Implementation Plan
Warehousing/Distribution
Whatever industry you work in, we can help you implement best practice Warehousing and Distribution Center (DC) solutions. This ensures your business is fully compliant with all necessary product traceability regulations while maintaining a high level of operational efficiency.
Our Strategy through Sustainability solutions include:
-
Warehouse/DC Automation
-
Warehouse/DC Management Systems (WMS)
-
Warehouse/DC Process Optimization
-
Serialized Operations Upgrades/Integrations
-
Edge/Scanning Integrations for Data Capture

Manufacturing/Packaging
Having installed, qualified, and serviced more than 200 packaging lines, we have the experience and expertise to help you implement the right Good Manufacturing Processes and solutions for your business.
Our team of subject matter experts and project managers can ensure you maximize operational efficiency while remaining fully compliant with all relevant regulations.
Our Strategy through Sustainability solutions include:
-
Line design, build and retrofit
-
Line installation and qualification
-
Manufacturing Execution Systems (MES)
-
Automation equipment
-
Aggregation technology
-
Overall Equipment Effectiveness (OEE)
-
Manufacturing contingency planning
-
Contract manufacturing
Healthcare Consulting
Our services help deploy DSCSA initiatives by ensuring alignment of their business strategy with their technology capabilities and regulations. With our focused approach and a flexible operating model, we strive to ensure full compliance and deliver improved program performance, with maximum value.
Are you DSCSA ready?
The Drug Supply Chain Security Act (DSCSA) became law in November 2013, providing a national standard for drug security and harmonizing existing state-level Pedigree regulations. DSCSA mandates fully supply chain traceability from pharmaceutical manufacturer to pharmacy dispenser, with compliance data exchange between supply chain partners at each change of ownership.