Our services help deploy DSCSA initiatives by ensuring alignment of their business strategy with their technology capabilities and regulations. With our focused approach and a flexible operating model, we strive to ensure full compliance and deliver improved program performance, with maximum value.
Are you DSCSA ready?
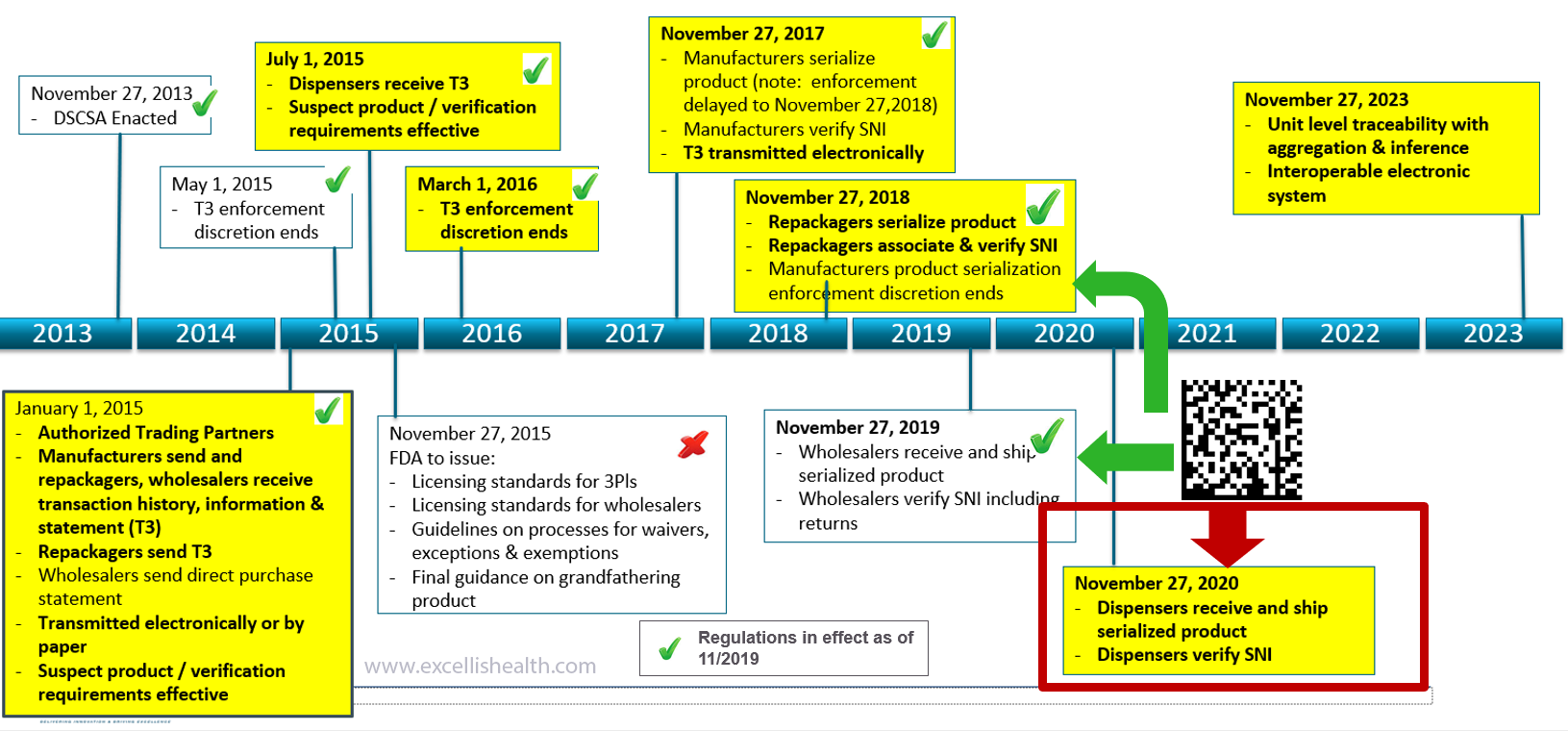
The Drug Supply Chain Security Act (DSCSA) became law in November 2013, providing a national standard for drug security and harmonizing existing state-level Pedigree regulations. DSCSA mandates fully supply chain traceability from pharmaceutical manufacturer to pharmacy dispenser, with compliance data exchange between supply chain partners at each change of ownership.
DSCSA Compliance Timeline – November 2020
White Papers
Our team of serialization, compliance, and project management experts offer a deep understanding of the latest supply chain trends, issues, and regulatory changes. Read a selection of our recent white papers here.
OIG Report: “Drug Supply Chain Security: Dispensers Received Most Tracing Information”
OIG Summary: “Drug Supply Chain Security: Wholesalers Exchange Most Tracing Information”
Upcoming Webinars
Stay tuned for our next healthcare/retail pharmacy webinar!
You can find our archive of past webinars here.
Global Track & Trace Dispenser Roundtable
Stay tuned for our next announcement!